 RenovaCare Announces Successful FDA Meeting
RenovaCare Announces Successful FDA MeetingSeccionesTVMore information from NBC Follow NBC News The FDA approves the first treatment of skin spray for burnsDylan Melancon estimates that it was 70 miles an hour when its bike crashed on a hot road in New Orleans this past June. She didn't wear leathers or protective clothing. The virtually unprotected body of Melancon slides along the concrete at high speed for the patios. "She wore pants and a shirt. Then, she didn't wear pants," said the 26-year-old nursing student. Melancon had deep burns and abrasions on his knees, elbows, thighs, stomach and back. They were the kind of injuries that usually put people in the hospital for months of painful skin grafts. Instead, Melancon's doctor obtained permission to treat it with ReCell, a sprayed skin product designed to treat burns with a patient's skin cell stain. "You just sprayed it in the patient, in the burned area," said Chris Houchens of the federal government's Biomedical Research and Development Authority, or BARDA, who helped pay for its development. The cells of the solution are attached and begin to grow, forming a fresh layer of normal skin. It was experimental then, but the Food and Drug Administration approved ReCell on Thursday night. Avita Medical, a small regenerative medicine company, will develop the product for sale in the United States. It is the first product of spraying skin to gain approval for the US market. Nearly half a million people get so bad burns that they need medical treatment, according to the American Burn Association and Centers for Disease Control and Prevention. RelatedThe current standard of care for serious burns is a skin graft. But the skin grafts mean to cut the healthy skin to put on burns, which results in more skin to heal. Besides, it's painful. "When they took the skin for the grafts, it burned like fire," Melancon said, who lives in New Orleans. Patients often end with defied scars in both the burned area and the skin from which the graft was taken. Scientists have been working for over a decade to invent something better. Spray skin is an obvious solution: take a small sample of skin, turn it into a solution, and cover the burned area with a thin layer of skin cells that can grow and cover the wound. RelatedTeams are working on 3-D skin printers, spray guns and other technologies to do this. Avita rival RenovaCare is working on a system called SkinGun that sprayed a similar fog of cells on a wound. Avita won the race for FDA approval with her ReCell system. To get the FDA approval, companies must appear with a unique technology and then show that it works at least as well as, if not better than, current treatments. The Avita system uses a unique combination of enzymes to decompose the skin layers of a piece of tissue, and then mix them into a liquid that can be applied to the skin by means of a low-tech spray syringe. The ReCell system considerably reduces the amount of skin that should be removed to place on the burnt surface, said Avita Medical CEO, Dr. Mike Perry. For a standard skin graft, surgeons should usually remove a healthy skin area not less than three quarters of the wound size, then stretch it over the damaged area. "So if a person has burns that cover 10 percent of his body, the area of the skin graft that would be required is almost equal to the full back of an older adult," Perry said. "You are creating new wounds when you take those skin samples to cover the burn." The most severe burns often cause little pain, because nerves are destroyed along with skin and muscle tissue. But grafts use the upper layers of the skin, right where the nerves are. "These patients have extraordinary pain," Perry said. "Taking those skin grafts is simply unbearable." The ReCell system significantly reduces the amount of healthy skin that should be removed, by 97 percent for a second-degree burn, Perry said. "If your entire back was burned, all you need is a piece of skin the size of a credit card to replace that damage," Houchens said. Burns heal faster and patients end up spending less time in the hospital, he said. Two clinical trials showed a faster recovery using less skin than standard grafts. It takes about 30 minutes to process a patient's skin sample, Perry said. Because cells come from the patient, there is no risk of rejection. Melancon was very happy with his results. "In general, I think it was 34 percent of my body that burned," he said. That would normally mean two months or more in the hospital for recovery, with much of that time spent taking narcotics for pain. Melancon spent only 3 1⁄2 weeks in the hospital after his 26 June accident. He said he stopped taking pain medications in mid-August so he could go back to his nursing classes. " On my knees I essentially burned to the bone," he said. He also lost the skin from areas of which skin grafts are often harvested: the back, the abdominal area and its thighs. "The doctor didn't have much skin to work with," Melancon said. He says he has very little pain. "I show all my grafts and scars and everyone is surprised how well it works," he said. capillary follicles have begun to grow in some areas, and in some, he said, scars are barely visible. "I'm proof it works," Melancon said. Perry said that Avita has not settled in a price for the ReCell system, but most likely it is between $5,000 and $10,000 per unit, which can treat about 10 percent of a patient's body surface. Someone with burns more than 50 percent of the body would require five units for treatment, more for deeper burns. BARDA will buy some of the kits for the national arsenal of medicines and equipment waiting for large emergencies, such as large explosions and chemical or nuclear attacks. "If you think of a massive casualty event, we don't have enough beds to treat hundreds of patients at once," Houchens told NBC News. "We don't have enough burn specialists. Our system will be completely overwhelmed. Having this technology that allows us to treat this in 30 minutes is really transformational." BARDA and other federal agencies have been trying to support and encourage researchers to make spray skin for years. The agency invested around $50 million in the development of ReCell." These products will be used mainly by hospitals and doctors to treat injured patients in a house fire or a gas explosion or a car accident," Houchens said. But, he said, when a company can commercially develop a government-backed product, it must achieve a wider use, which can reduce the price. 2021 NBC UNIVERSAL
Lab-Grown Skin as good as skin grafts for burns in Phase IIIMallinckrodt treatment made of artificial skin tissue called Stratagraft closed more than 80% of the second-degree burn wounds in a phase III test, which coincides with the effectiveness of the skin grafts. The treatment of Mallinckrodt made of artificial skin tissue called Stratagraft closed more than 80% of the second-degree burn wounds in a phase III test, coinciding with the effectiveness of the skin grafts. Mallinckrodt recruited 71 patients with second-degree burns that were eligible for skin grafts in the trial. Each patient received artificial skin grafting in some areas of burnt skin, and a regular skin graft in others as a control. Three months later, artificial skin closed 83% of the burn wounds, which was as effective as 86% of the burn wounds closed with regular skin grafts. With this evidence in hand, Mallinckrodt plans to apply for FDA approval in 2020. Mallinckrodt when he purchased the American company Stratatech Corporation in 2016. Treatment outside the platform is done by cultivating skin cells in the laboratory to form layers of tissue that mimic human skin. The artificial skin is tied to burn wounds with staples, sutures or surgical glue. A recent one showed that Stratagraft eliminated the need for skin grafts, which means taking healthy skin from a different part of the patient's body. "The second wound created by the elimination of healthy skin can be associated with complications and can be even more painful than the burn wound itself," said James H. Holmes, co-liter researcher of phase III trial, and Director of Wake Forest Baptist Medical Center's Burn Unit, USA. Multiple companies are developing regenerative medicines to replace the need for the skin donated in the treatment of burns. For example, the New Zealand company Upside Biotechnologies intends to take patient skin cells and grow them in . This would especially help patients who do not have enough healthy skin to provide a normal graft. The researchers in Spain have also developed one that could make human skin graft materials in the future. they are a special approach to burning research. For example, the American company Renova Care is developing one that can shoot cells on burns to accelerate the healing process. Shutterstock Images Explore Related Topics: More from LabiotechLabiotech.eu is the main digital media that covers the European Biotech industry. More than 150,000 monthly visitors use it to monitor business and innovations in biotechnology. I hope you enjoy reading our stories! Items for Members

Our Technology | RenovaCare
New Burn Healing Method uses Skin-Gun Stem-Cell Therapy | Digital Trends
Skin Rejuvenation Machine Vanadium Titanium Beauty Instrument Meso Gun for Wrinkle Removal/Moisturizing/Whitening/Skin Tightening FDA Approved: Amazon.ca: Beauty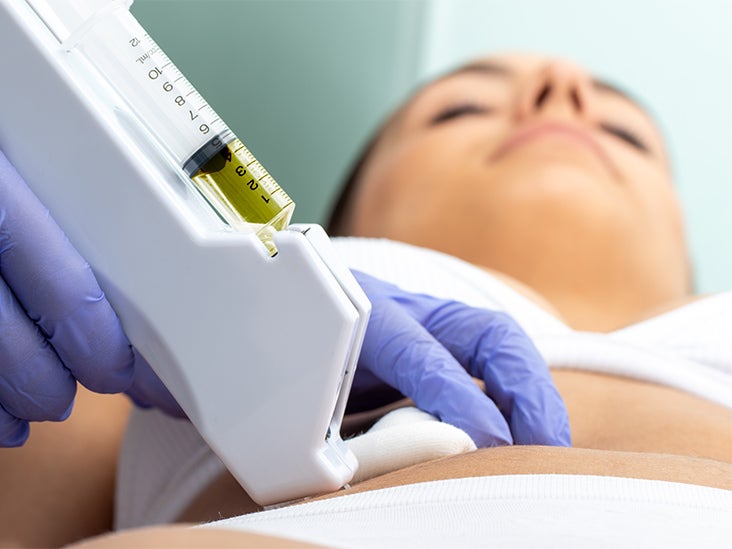
Skin Cell Gun for Burns: How It Works and Where It's Available
Skin Rejuvenation Machine Vanadium Titanium Beauty Instrument Meso Gun for Wrinkle Removal/Moisturizing/Whitening/Skin Tightening FDA Approved: Amazon.ca: Beauty
Vanadium Titanium Facial skin care machine, Skin Rejuvenation Machine Mesoderm Gun Wrinkle Removal Skincare Whitening Tools FDA Approved | Walmart Canada
Skin cell gun sprays stem cells for fast recovery from serious burns | RobAid
Our Technology | RenovaCare
Skin cell gun sprays stem cells for fast recovery from serious burns | RobAid
Australian developed spray-on skin for burns treatment seeks FDA approval | Healthcare IT Australia
FDA approves first spray-on skin treatment for burns
This New 'SkinGun' Helps Burn Victims Regrow Their Skin in Just a Few Days![Skin Gun]()
Skin Gun" Uses Autologous Skin Cells to Heal Severe Burns in Days, Not Weeks | Medgadget
FDA approves first spray-on skin treatment for burns
Fat Iron Is The 1st FDA Approved Iron That Reduces Body Fat And Tightens Skin
Pin on Skin Care
Which Products Need FDA Approval? | Products-Liability-Insurance.com
This spray-on nanofiber 'skin' may revolutionize wound care
FDA OKs new indications for Icecure's cryoablation technology, new Multisense system | 2020-01-07 | BioWorld
China FDA Approved Skin Cooling Sterile Derma Roller 540 Micro Needle - China Roller, Derma Roller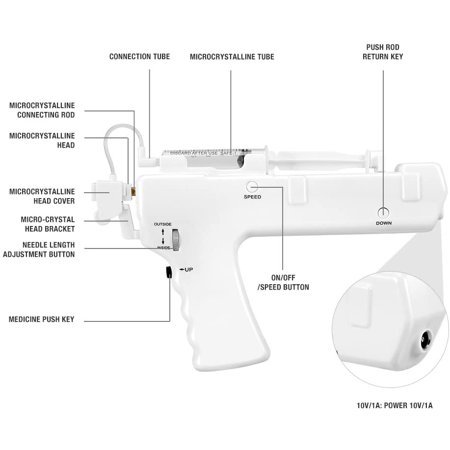
Vanadium Titanium Facial skin care machine, Skin Rejuvenation Machine Mesoderm Gun Wrinkle Removal Skincare Whitening Tools FDA Approved | Walmart Canada
FDA Approves First Spray-On Skin for Burn Treatment
The Dermal Regenerator
Ivermectin - FDA prescribing information, side effects and uses
FDA Approves World's First Pill with an Embedded Sensor | designnews.com
New Technology Could Help Burn Victims Regrow Skin for a Faster Recovery
Professional Metallic Ear Piercing GUN (with Skin friendly 'Viscot' Skin Marker, carrying case, 24 Metal Studs, with both Rubber & Metal stopper): Amazon.in: Health & Personal Care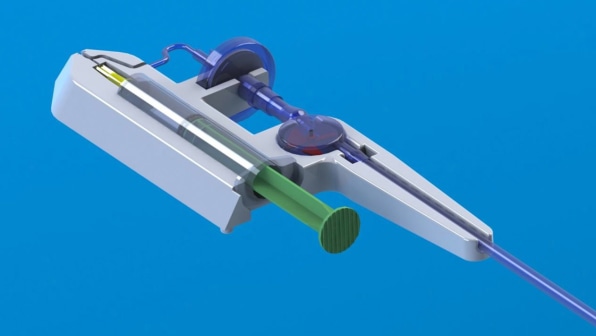
The SkinGun Sprays Wounds With Your Own Skin Stem Cells For Faster Hea
Groundbreaking Israeli Medical Device Treats Burns Without Ever Touching The Patient
Skin-cell spray gun drastically cuts healing time for burns
CoolSculpting for Your Arms Is Now a Thing, Too | Allure
RenovaCare Device Delivers Stem Cells to Grow New Human Skin | mddionline.com
Ce FDA Approved Good Quality Digital Non Contact Infrared Forehead Thermometer with Fast Shipping | Global Sources
When should the public expect an FDA-approved vaccine for kids?
Pin on Skin Care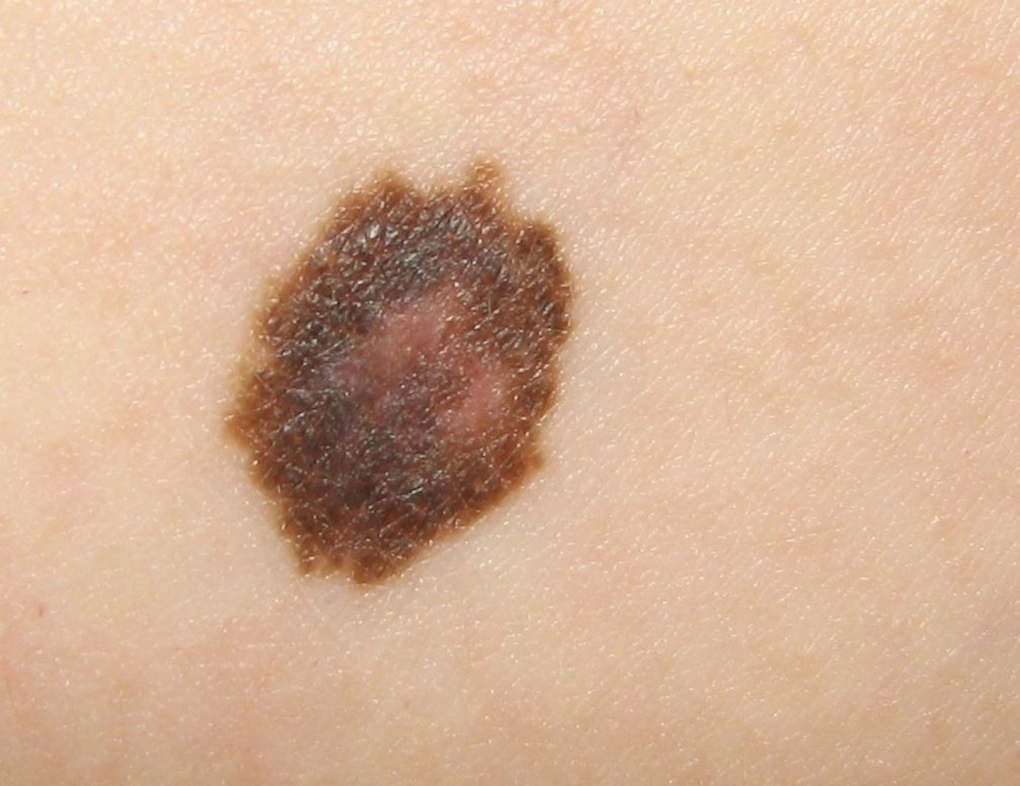
FDA approves new kind of cancer drug for advanced melanoma | CTV News
FDA approves first spray-on skin treatment for burns
Radiesse gets FDA approval for hand rejuvenation - New York Daily News
This New 'SkinGun' Helps Burn Victims Regrow Their Skin in Just a Few Days
Spray-on nanofibre 'skin' treats burns and wounds - TODAY
 RenovaCare Announces Successful FDA Meeting
RenovaCare Announces Successful FDA Meeting



































Posting Komentar untuk "skin gun fda approval"